Medical Molder Sweats the Small Stuff to Deliver Big Results
Embracing lean manufacturing and scientific molding keeps Freudenberg Medical on top of customer demands for defect-free deliveries as it expands into full device manufacturing, 100% automated inspection, and 3D-printed tooling for quicker product launches.
“You think you know everything—but when you write it down, you see the small things,” explains Rao Neelam, v.p. of operations at Freudenberg Medical’s Baldwin Park, Calif., medical-device manufacturing facility. The “small things” are a big deal at Freudenberg Medical’s operation approximately 20 miles east of Los Angeles. The facility serves as a thermoplastic injection molding lead center for Freudenberg Medical’s 12 manufacturing sites spread across the U.S., Costa Rica, Ireland, Germany, and China.
As a lead center, Baldwin Park is the primary residence for all the company’s thermoplastic injection molding technical expertise, developing and disseminating best practices for all of Freudenberg Medical’s molding facilities, located in Gloucester, Mass., China, Germany, and Costa Rica.
The “small things” Neelam is talking about are potential “undesirable effects” that have been written along one axis of a chart spread across multiple sheets of paper. These charts are taped to the wall of the main corridor in Baldwin Park, which acts as the primary artery for the facility, running its length and separating the company’s various operations.
On the chart’s other axis, employees have written out “root causes” for the aforementioned “effects.” All of this is part of a value-stream mapping operation undertaken continuously at Freudenberg Medical as part of its deeply ingrained lean-manufacturing philosophy. At least twice a month, a cross-functional team undertakes a deep dive into a particular process, mapping out any potential bad outcomes and how they could occur, as a means to avoid them.
“Lean” is very much a part of Freudenberg Medical’s corporate culture, and its implementation internally is maintained via a more than 30-yr-old company initiative known as GROWTTH (Get Rid of Waste Through Team Harmony).
“We have a very robust lean program here,” says Ward Sokoloski, v.p. and general manager of Freudenberg Medical. “By utilizing lean tools, we’re able to get more out of the space that we currently have available.” Sokoloski explains that Baldwin Park has been able to implement one-piece flow throughout its processes, generating new floor space within its clean rooms and allowing the company to run more operations out of the existing footprint, which is a money saver in the very expensive Southern California real-estate market.
The Baldwin Park operations cover 72,000 ft2, with 26,000 ft2 of that made up of Class 7 and Class 8 clean rooms. Apart from the recently expanded tool room, all of Baldwin Park’s operations—including inspection, assembly, decoration, post-mold curing/ annealing, and packaging—occur in a clean-room environment. When Plastics Technology visited, the plant was running around 60 Arburg, Inc. and Toyo injection machines, from 28 to 350 tons, with a new Arburg on order. Baldwin Park has three two-shot machines with rotating platens, in vertical and horizontal configurations. There are close to 200 employees on staff, and the plant runs three shifts, 24/7. The plant is 100% devoted to medical molding and has ISO 13485 certification. In 2015, it completed FDA registration to move into production of several types of complete medical devices rather than only components.
“Because California is such a wonderful area in which to grow the medical business, due to the large number of medical OEMs present here, we felt registration was a great opportunity to offer that device service here in Baldwin Park,” Sokoloski says.
100% INSPECTION
The trend toward miniaturization in medical, driven by minimally invasive surgeries where incisions are measured in millimeters instead of inches, is evident at Freudenberg Medical in the precision required by its customers. For one job, the accuracy requirements led Baldwin Park to install a 100% inspection system on a line running to 10 million parts annually.
Instead of an agreed-upon AQL (acceptable quality limit) sampling plan, where, for example, every four hours an inspector collects and examines parts, including critical dimensions, 100% automated inspection means just that: Every single part is completely inspected.
“We think 100% automated inspection is where our business is heading,” Sokoloski says. “We’ve already installed one line, but the more customers we talk to—especially in critical applications and high-volume applications—they’re the ones that are really expressing more interest.”
By identifying a defective component the moment it’s made, Freudenberg Medical can avoid deeper disruptions to its own and its customers’ production. “If a bad part goes to the customer and the customer sees it, then it’s a whole different scenario,” Sokoloski says. “The customer has to stop its line, send the part back, and we have to stop our line. But if we catch it immediately, we don’t even produce the next part; we fix it immediately.”
Even before outfitting a production line with visual inspection equipment, Baldwin Park operated a full metrology lab to track critical dimensions on existing and upcoming projects. For new projects, the company performs full first-article inspections, checking every dimension and developing a measurement systems analysis (MSA) program that’s sent to the production floor. The adherence to precision is necessitated by the tolerances Neelam and his team are asked to maintain: ±2 microns (0.0000787 in.) on credit-card-sized parts, for example.
“Matching the 2 microns is the biggest challenge,” Neelam says. “Making a part is one thing, but also being able to measure and maintain that is the challenge.”
In addition to molding and maintaining that level of accuracy, Freudenberg Medical is also tasked with creating complete transparency in its operations. Utilizing an SAP ERP system that’s standardized throughout its operations, it can trace everything about a part from the lot of material used in its manufacture to the operator and machine that produced it, the cavity it came from, and who did final inspection. This supports the FDA’s 2013 “final rule” on a unique device identification (UDI) system.
“Everything is traceable,” Sokoloski says. “That’s what we’re seeing with the UDI. We’re now having to get down to individual traceability at the device level.” At Baldwin Park, this has manifested itself in the installation of laser-marking equipment, which is used to give an ID to every component that comes off a machine.
Sokoloski also stressed that Freudenberg Medical does not inspect quality into its products; it builds quality into its production via the installation qualification, operational qualification, and performance qualification (IQ/OQ/PQ) it under- takes with all new projects. “The trend we see is that even with IQ/OQ/PQ and six-sigma quality, defects are still created when producing millions of parts,” Sokoloski says. “To a customer, a single defect is not acceptable. Therefore we are providing our customers with the added service of 100% automated inspection—in addition to a validated process.”
EXPANDING A TOOL ROOM, THE ‘LEAN’ WAY
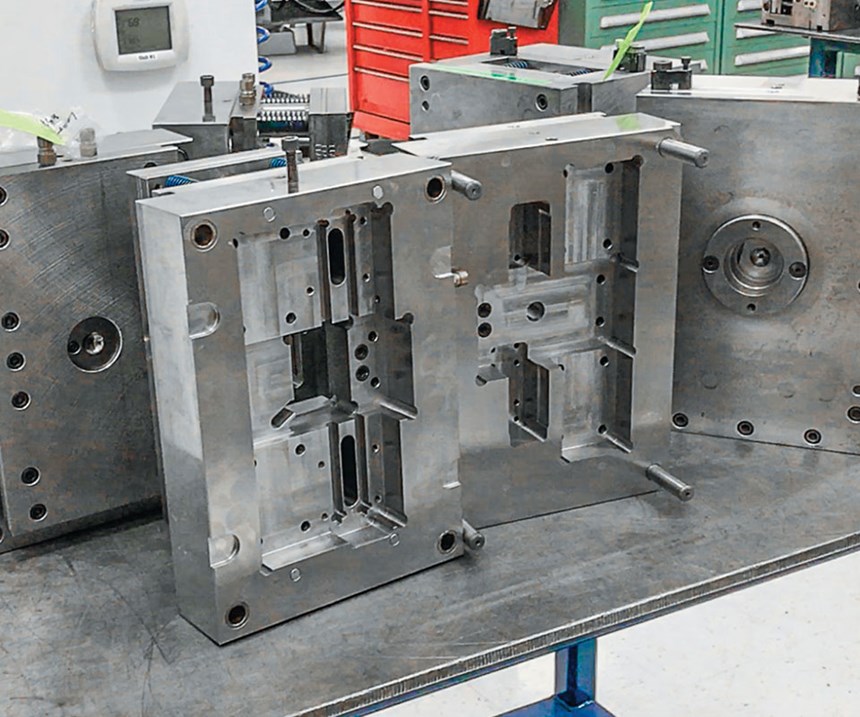
In order to maintain and repair molds in a timely manner, Baldwin Park expanded its tool room, completing the installation of equipment at the start of this year. The new equipment was installed in the enlarged space only after Neelam and his team completed a lean 3P (production preparation process) study to determine the most efficient way to configure it in the given space.
“You’re basically planning for manufacturing,” Sokoloski says of the 3P study. “How do you design and lay out the operation so that it flows with minimal waste?” So Neelam and his team looked at the various pieces of equipment; they identified which pieces would get the most use; they looked at the transit time and the motion to go from one piece of equipment to another; and laid out the entire tool room in a lean fashion.”
The tool room’s primary function will be preventive maintenance. Neelam estimates that Baldwin Park has around 150 active molds, with roughly 200 that are more “seasonal.” When PT visited, about 70 new tools were being validated, giving the tool room plenty to do going forward. Like most operations at Baldwin Park, the facility’s tool management program is extremely detail-oriented. Each mold has a unique code and dedicated home on the tool-storage racks, with maintenance scheduled on the basis of production cycles.
By using a four-tag system—green, yellow, red, and white—workers can visually ascertain a tool’s status. A white tag means a mold has just finished a production run and is ready for storage after a complete cleaning. A red tag indicates something’s wrong, and the mold requires some work. Damaged tools receive a yellow tag, while green means production-ready. After cleaning, tools are wrapped in plastic and their ID number is written in marker on the outside. When tools head to production, they are accompanied by a plastic bin, bearing the same number, that holds all the water lines, sprue bushings and other components that belong to that tool.
“We keep the exact length of hose for each mold,” Neelam says, noting that the proper length is determined during process validation. “It’s very critical to maintain the same conditions all the time.” For high-volume tools, Freudenberg Medical keeps an inventory of spare parts for wear components. The location of these spare components is mapped down to the exact drawer in the exact cabinet within the tool room.
RAPID PARTS FROM RAPID CAVITIES
In July 2016, Freudenberg Medical announced its Rapid Prototype WorkCell to speed product launches. At the heart of the program are custom 3D-printed cavity inserts that can be made in two to four days, depending on complexity. These printed cavity inserts are dropped into modular mold systems and run on dedicated machines by a dedicated team.
There are six different types of mold bases into which the cavities, printed from ABS, can be installed. In addition to a basic base with no movements, Freudenberg Medical has one with slides running lengthwise; one with slides on all four sides; one with unscrewing for threaded parts; and one with a hot- runner valve-gate system.
“If you want to actually assemble parts and test them for functionality, you cannot do that with printed parts,” Neelam says, “but these are real molded parts, so you can do the functional testing.”
EXPECTATIONS OF 100%
Freudenberg Medical’s Baldwin Park facility was launched 20 years ago in 1997 as Anura Plastics Engineering Corp. (APEC), taking its name from one third of the founding partnership of Anura Welikala, Wolfgang Buehler and Mark Bonifacio. In the intervening years, including its 2008 acquisition by Freudenberg, it has remained dedicated to medical molding and the attention to detail it requires.
“We are going to stay 100% medical in our vision and our strategy,” Sokoloski says. “We recognize that customers are expecting 100% good parts, every single time. Even though there might be a sample plan in place, the expectation is 100% good parts.”
Related Content
Consistent Shots for Consistent Shots
An integral supplier in the effort to fast-track COVID-19 vaccine deployment, Retractable Technologies turned to Arburg and its PressurePilot technology to help deliver more than 500 million syringes during the pandemic.
Read MoreIPEX Opens Injection Molding Facility in North Carolina
The pipe and fittings manufacturer’s new 200,000-square-foot facility represents a $200 million investment and will create 150 jobs.
Read More50 Years...600 Issues...and Still Counting
Matt Naitove marks his first half-century in plastics reporting, with a few of his favorite headlines.
Read MoreSlimmer All-Electric Press Debuts
NPE2024: A slimmed-down version of Engel’s all-electric, e-mac injection molding machine is among eight displays, which also include LSR micromolding, quick mold changes and a cube mold.
Read MoreRead Next
How Serious Is Freudenberg Medical About Lean Manufacturing?
One employee I met there helped his daughter undertake a Kaizen event on her closet.
Read MoreRecycling Partners Collaborate to Eliminate Production Scrap Waste at NPE2024
A collaboration between show organizer PLASTICS, recycler CPR and size reduction experts WEIMA and Conair will seek to recover and recycle 100% of the parts produced at the show.
Read MorePeople 4.0 – How to Get Buy-In from Your Staff for Industry 4.0 Systems
Implementing a production monitoring system as the foundation of a ‘smart factory’ is about integrating people with new technology as much as it is about integrating machines and computers. Here are tips from a company that has gone through the process.
Read More






















 (2).jpg;maxWidth=300;quality=90)